Does Your Ruby Glow?
A woman is enjoying the booths at a local Gem & Mineral show. She’s looking at some pretty pendants done by local lapidary artists when the gentleman manning the booth says “That’s a beautiful ring you’re wearing! Is it a ruby?” The woman looks down at her hand, and looks at the ring her sister gave her, a round, faceted beautiful red stone in a traditional gold setting. “Yes, it is. My sister gave it to me as a graduation present!” The man looks at her and in a serious tone says, “I can test it, to see if it’s a real ruby”. Surprised but intrigued, the woman agrees. He shows her a black box. “This”, he says, “will shine UV light on your ring. If it’s real, it will fluoresce a bright red color”. She hands him the ring and he puts it in the box. He then looks through the eyepiece and turns on the switch. “Oh no!”, he says “It doesn’t fluoresce. It’s just staying dark”! The woman is shocked! “You mean it’s not real?” she exclaims.
A ruby ring in yellow gold.
Was her stone real? Does this test prove that the ruby isn’t real? Did her sister get cheated at the jewelry store where she bought it?
She needn't worry, it’s real, and the fluorescence test did not entirely mislead her. Rubies do fluoresce under UV light…just not all rubies. The fluorescence characteristic of rubies has been known for a very long time. It is touted by some as a test for “real” rubies. The issue is that it only works for some rubies, so it’s not a definitive test. Let’s delve into what’s actually happening.
All gemstones have a specific chemical composition. Sometimes, while the crystals grow, other atoms, that aren’t part of this essential chemical makeup become incorporated into the gemstone. These atoms, present only in minute amount, are called trace elements. Rubies and sapphires are both varieties of corundum. Corundum’s chemical composition (Al2O3) consists of only aluminum (Al) and oxygen. When certain trace elements become incorporated, the result is different gem colors. Chromium (Cr), for example, imparts the gorgeous red of ruby, while iron (Fe) + titanium (Ti) the beautiful blue of sapphire. [Historically, gems were classified by color. So, even though today we know they are the same gem species (corundum), rubies & sapphires are still identified separate gemstones. All other colors are labeled fancy sapphires.]
19.53 Carats Burmese Star Ruby Pendant Necklace at auction (Christie's Hong Kong). Created by jewellery designer Edmond Chin for Etcetera.
Top-quality ruby is perhaps the world’s most expensive gemstone, and the finest grade rubies are more valuable than equivalent-sized flawless colorless diamonds. The finest ruby has a pure, vibrant red to slightly purplish red hue (if the red is too light it is called a pink sapphire).
Rubies and sapphires form under specific temperature and pressure settings where the geologic environment has a low silicon content. Primary deposits are found in either magmatic or metamorphic geologic terrains. For rubies to form, not only must there be very little silica, but there must also be a source of Cr. In some environments, rocks with high Cr content also have iron (Fe). This results two groups of rubies: low-iron and high-iron rubies.
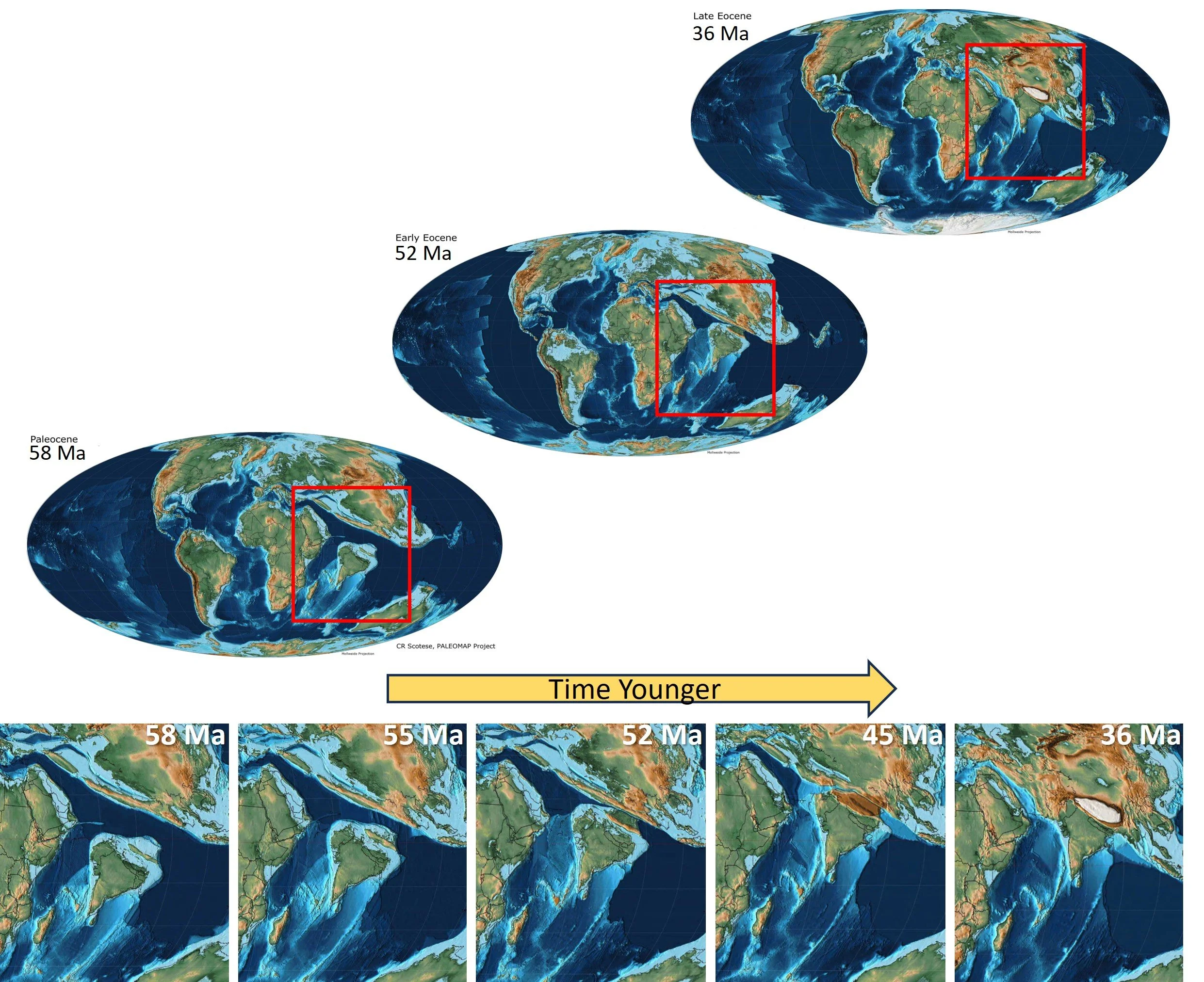
Low-iron rubies are also called marble-hosted. They are found in locations in which limestone has been metamorphically changed to marble. These rocks typically have very little iron. The marble-hosted group encompasses rubies from the legendary ruby mines in Burma (Myanmar) as well as Vietnam, Tajikistan, and Afghanistan. The deposits were all formed during the Himalayan orogeny (40 - 50 million years ago, or Ma), during which the Indian and Eurasian plates collided - compressing, burying, and subducting the platform carbonates (limestones) which had formed in the warm shallow sea between them.
Paleogeography showing the collision of Indian and Eurasion plates resulting in the Himalayas (maps from C. Scotese, PaleoMap Project, 2014).
Marble hosted ruby from Yen Bai Province, Vietnam. Alfie Norville Gem & Mineral Museum, Arizona. Loaned by Mark Lefont. Photo author's own.
Exposed to extreme temperatures and pressures during this collisional event, the limestone transformed into marble. In the meantime, fluids circulating through the rocks mobilized aluminum and chromium, and all the key ingredients for the formation of rubies came together.
High iron ruby and Ruby-Zoisite specimens in Tanzanian gemstones display at GIA HQ in Carlsbad, California. Photo author's own.
High-iron rubies on the other hand occur across the globe and from many different geologic ages from the Archean (2.97–2.6 billion years ago, or Ga) to the Cenozoic (65 Ma to present). Rubies in this group derive from metamorphic, metasomatic, or intrusive events, but are all associated with ultramafic igneous rocks, from which they derive both Cr and Fe.
Rubies with no Fe have a pure red color which fluoresces under sunlight and longwave UV light. On the other hand, high-iron rubies tend to have a darker color and suppressed fluorescence, both of which are caused by the Fe. There is a prevailing myth that “real” rubies will fluoresce under ultraviolet (UV) light. Any ruby that has little to no Fe will fluoresce, including (and most especially) synthetic rubies. Pink sapphires will also fluoresce because they contain Cr, just not enough to be categorized as "rubies". Fluorescence does not guarantee a “real” nor a quality ruby. It only tells you the stone has Cr and little to no Fe.
Synthetic ruby showing strong fluorescence, Alfie Norville Gem & Mineral Museum, Tucson, Arizona. Donated by Raymond Mayson, 2019. Photo author's own.
To understand this interesting phenomenon, let’s look at how light interacts with rubies and specifically these two trace elements.
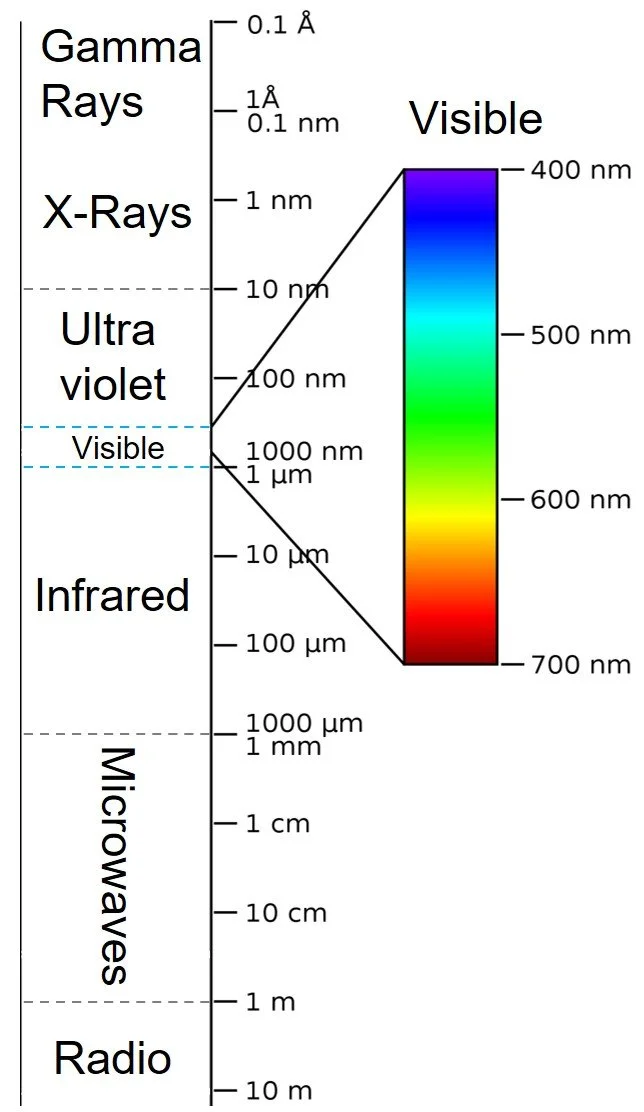
Humans see color when light is either transmitted or reflected by an object. The object must, in some way, take white light, which is the combination of all spectral colors, separate the different colors (wavelengths), and only transmit or reflect certain ones. The visible spectrum (that which we can see), is essentially a rainbow. Each color is a component of the white light with its own wavelength and energy. The shorter the wavelength, the higher the energy. So, violet has more energy than red. Ultraviolet (UV), which we cannot see, is even higher energy than violet.
Electromagnetic Spectrum. Note visible light is only a small portion.
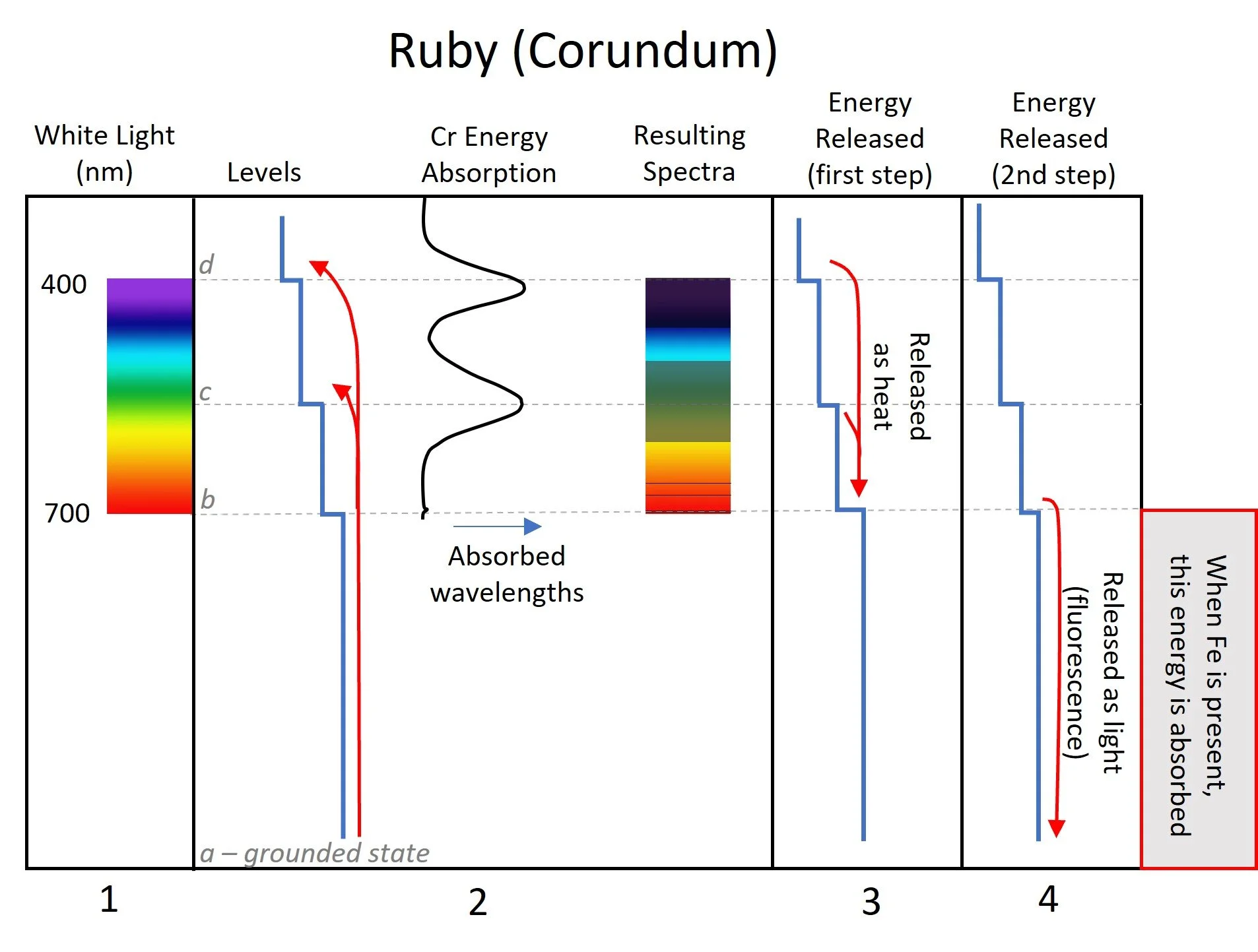
When light hits a ruby, the full visible spectrum enters the stone (Figure Stage 1). Cr impurities in the stone absorb yellow, most of the green, and the violet portions of the color spectrum. What’s left, and what we then see, is red, orange, and some slight blue. This pattern, called the absorption spectrum, can be viewed using a spectroscope and is key in identifying gemstones with Cr, like rubies (Figure Stage 2). Cr can also cause red fluorescence which, under daylight, adds to the intensity of the red color.
White light interacts with chromium in ruby, resulting in a characteristic absorption spectrum. See text for explanation of steps 1-4. After K Nassau
The absorption spectrum has this characteristic pattern because the incoming radiation (energy) is absorbed by the Cr ion and this allows its electrons to jump to a higher valence state, which you can imagine as an orbit level. These higher levels are at specific distances from the nucleus and a specific amount of energy is required to reach them. For Cr3+ ions, those specific energy levels correspond exactly to the wavelength energy of the colors that are absorbed (Figure Stage 2).
Eventually (very soon actually), those electrons fall back down to lower levels. As they move lower, they release the energy back into the gemstone. Mostly, this energy becomes heat (Figure Stage 3). However, when the electron moves from the last level back to the stable state, it releases light. This light is the fluorescence we see (Figure Stage 4).
Fe is also an impurity that can also create color in gemstones, but often does so by a slightly different mechanism. In the case of rubies that contain both Cr and Fe, the rubies are still red, and they still show the characteristic absorption spectrum of Cr (with some modification). But, they do not fluoresce. The reason for this is that the energy released by the electrons slipping back to the stable state (Figure Stage 4) is absorbed by the Fe and so light is never released.
Good quality ruby has a pleasing, medium tone, saturated red color. They very often have inclusions, but and the fewer or less noticeable they are, the higher the value of the stone. Although historically, rubies came from the metamorphic marble terrains of central Asia, they don’t only come from there. Today, many desirable rubies come from Thailand, Cambodia, Madagascar, Mozambique, Tanzania, Kenya, and Greenland – all high iron localities. They are real, they just don’t fluoresce! It’s likely the ruby in the woman’s graduation gift came from one of the high iron localities and she can continue to enjoy her gift for many years to come!
Examples of both marble hosted (Cr) and high-iron (Cr + Fe) rubies. After C.Smith, 2008.
References
Aaron C. Palke, et. Al, 2020, Geographic Origin Determination of Ruby, Gems & Gemology, Vol. 55, No. 4, pp. 580–612, http://dx.doi.org/10.5741/GEMS.55.4.580
Awasthi, Neeraj and Ray, Jyotiranjan, 2020, The Palaeogene record of Himalayan erosion in the Andaman Basin, Journal of Earth System Science 129 15, Indian Academy of Sciences https://doi.org/10.1007/s12040-019-1266-7
Chao Liu, et al, 2017, Chromium mineral ecology, American Mineralogist, Volume 102, pages 612–619
Crystal Field Theory, https://en.wikipedia.org/wiki/Crystal_field_theory
Emily V. Dubinsky, Jennifer Stone-Sundberg, and John L. Emmett, 2020, A Quantitative Description of the Causes of Color in Corundum, Gems & Gemology, Vol. 56, No. 1, pp. 2–28,
http://dx.doi.org/10.5741/GEMS.56.1.2
Gaston Giuliani and lee A. Groat, 2019, Geology of Corundum and Emerald Gem Deposits: A Review, Gems & Gemology, Vol. 55, No. 4, pp. 464–489, http://dx.doi.org/10.5741/GemS.55.4.464
Nassau, Kurt, 1978, The origins of color in minerals, American Minerologist, Volume 63, pages 219-229, http://www.minsocam.org/MSA/collectors_corner/arc/color.htm
Nassau, Kurt, 2001, The Physics and Chemistry of Color, Wiley-Interscience, 496p.
Scotese, C.R., 2014, The PALEOMAP Project PaleoAtlas for ArcGIS, version 2, Volume 1, Cenozoic Plate Tectonic, Paleogeographic, and Paleoclimatic Reconstructions, Maps 1-15, PALEOMAP Project, Evanston, IL.
Smith, Christopher P, 2008, The Burma Ruby Ban and Alternate Ruby Sources, American Gemological Laboratories, Research & Publications, https://www.aglresearch.com/research-publications/ban-burma-ruby